




Next: Electrons as de Broglie
Up: Bohr model
Previous: Bohr model
de Broglie waves
In the early 1900s there were two broad theories of nature - Maxwell's theory
of electromagnetism and light, and Newton's laws for the motion of
particles. Some scientists from a philosophical viewpoint didn't like
this separation, and were looking for connections between the two
general theories. One scientist, de Broglie, proposed that, like
light, ordinary particles could exhibit wave-like properties.
He furthermore wrote down a relation between the wavelength of a wave
which was to be associated with a particle:
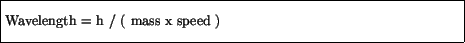
where ``h'' is a constant called Planck's constant:
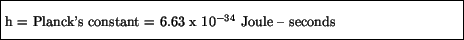
Such waves are now called de Broglie waves.
modtech@theory.uwinnipeg.ca
1999-09-29
![]()
![]()