



Next: Radioactivity
Up: Nuclear Physics
Previous: Nuclear Physics
Atoms in nature generally are electrically neutral, as they have equal numbers
of protons in the nucleus and orbiting electrons. However, within the nucleus
there are other particles called neutrons, which are electrically neutral but
have about the same mass as protons. There are two numbers used to
characterize a nucleus:
-
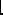 Z
Z
- , the atomic number, which equals the number of
protons;
-
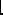 A
A
- , the mass number, which equals the number of nucleons
(protons and neutrons).
An element X is defined by the atomic number Z , while A denotes
the particular isotope of that element. The usual notation for an element
X is AZ X - for example, there are four common isotopes of
Carbon:
116 C ,
126 C ,
136 C , and
146 C , with
126 C being the most abundant ( > 98% ).
It is convenient in some circumstances
to measure masses in terms of the unified mass unit, u,
which is defined so that 12 C has a mass of 12 u exactly;
in SI units,
|
1 u = 1.66 x 10- 27 kg .
| (1)
|
There is also another convenient unit of mass which arises from Einstein's
Special Theory of Relativity. We have not covered this in this course,
so we simply quote the relevant (well-known) relation:
which associates an energy E to a mass m , with
c = 3.0 x 108 m/s
being the speed of light. Thus, dimensionally, E/c 2 is a unit of mass.
It is customary to express this unit in terms of MeV/c 2, where
1 MeV=10 6 eV=
1.6 x 10- 13 J - note that ``c'' here
is considered part of the unit, and one does not
substitute the numerical value
of
3.0 x 108 m/s in it. This will be illustrated later in some examples.
Through this relation one can find the energy equivalent
of 1 u:
|
E
|
|
= (1.67 x 10- 27 kg )(3.0 x 108 m/s )2
| |
|
|
|
= 1.50 x 10- 10 J = 9.39 x 108 eV = 939 MeV ,
| (3) |
which is then written as
One important illustration of the equivalence of mass and energy
of Eq. (29.2) has to do with what is called the
binding energy of the
nucleus. It is observed that the mass of any nucleus is always less
than the sum of the masses of the individual constituent nucleons which
make it up. This ``loss'' of mass which then results when nucleons
form a nucleus is attributed to a ``binding energy'',
and is a measure of the strength of the strong force holding the
nucleons together.




Next: Radioactivity
Up: Nuclear Physics
Previous: Nuclear Physics
www-admin@theory.uwinnipeg.ca
10/9/1997
![]()
![]()
![]()
![]()