



Next: General Properties of Waves
Up: Wave Properties of Light
Previous: Wave Properties of Light
- In vacuum light always travels at the same speed:
c = 3.0 x 108 m/s .
- Until the middle of the 1800's, the generally accepted theory
of light was the particle picture. In this viewpoint, advocated by
Newton, light was considered to be a stream of tiny particles.
However, in the late 1800's, the particle picture was replaced by
the wave theory of light. This was because certain phenomena
associated with light, namely refraction, diffraction and
interference, could only be explained using the wave picture.
-
Visible light is just one particular type of electromagnetic
radiation. Other types of electromagnetic radiation include
radio waves, infrared radiation (heat), ultraviolet radiation,
x-rays and
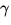 -rays. The different types of radiation are distinguished
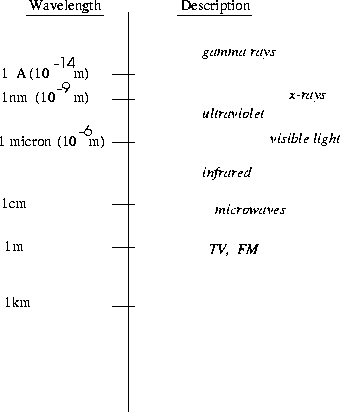
by their wavelength, or frequency, as shown in Fig. (22.1).
-rays. The different types of radiation are distinguished
by their wavelength, or frequency, as shown in Fig. (22.1).
Figure 22.1:
The Electromagnetic Spectrum
 |
For
example blue light has a wavelength (in vacuum) of
434 x 10- 9m = 434 nanometers (nm), while red light has
a wavelength of 768 nm. Radiation outside the visible spectrum
with wavelengths longer than red light is called infrared, while
radiation with wavelength shorter than blue is called ultraviolet.
The theory which accurately describes the wave-like properties of
all types of electromagnetic radiation is called Maxwell's Theory
of Electromagnetism.
- In the early 20th century, experiments revealed that there
were some phenomena associated with light that could only be
explained by a particle picture. Thus, light as it is now
understood, has attributes of both particles and waves. In this
Chapter we will deal mainly with the wave attributes
of light. The particle-like behaviour of light is described by the
modern theory of quantum mechanics, which will be described in
Chapter 28.




Next: General Properties of Waves
Up: Wave Properties of Light
Previous: Wave Properties of Light
www-admin@theory.uwinnipeg.ca
10/9/1997
![]()
![]()
![]()
![]()