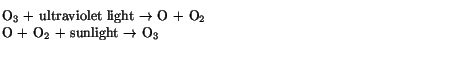
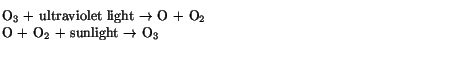
It was only after this protective shield was formed in the upper atmosphere that life evolved from the oceans to land.
However, the introduction of CFCs (chlorofluorocarbons) has led to a partial destruction of the ozone layer. CFCs were introduced in the 1950s as an alternative to toxic chemicals such as ammonia that were used as refrigerants in air conditioners and refrigerators and in aerosol sprays. Being fairly non-reactive, they were seen at the time as a major breakthrough in this technology. However, it was later realized that the chlorine (Cl) present in CFCs does react with the ozone in the upper atmosphere, according to the following reaction:
![]()
This destruction of ozone poses a risk to the Earth in the form of increased exposure to ultraviolet radiation and the resulting dangers of, for example, skin cancer and decreased crop yields.
The destruction of the ozone layer was first noticed in the late 1980s as a hole over Antarctica. The reason that the destruction is so prominent there is that during the winter months, when no sunlight reaches the area, chlorine builds up, and then during the spring the sudden presence of sunlight induces a large number of ozone-destroying reactions. This leads to a noticeable ``hole'' in the ozone layer above Antarctica during the summer. After this initial discovery, however, it was soon detected that the ozone layer is thinning out on a global scale.
This problem has been addressed in 1986 in Montreal where most of the world's countries agreed to phase out CFCs by the late 1990s. The major stumbling block to this agreement was the acknowledgment by the developed countries that the developing nations would need financial assistance to implement this agreement and phase in the alternatives to CFCs. Although it will take some decades for the ozone layer to return to normal levels, it is generally thought that this problem, and its solution, were addressed in time in order to avoid permanent damage.