




Next: Wave particle duality
Up: Bohr model
Previous: Absorption spectrum
Stimulated emission
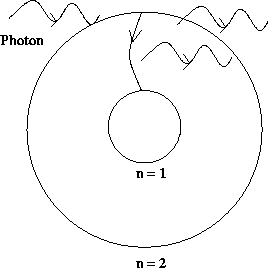
A third type of photon-related electron transitions in an atom is
stimulated emission. Suppose an electron is in a higher
energy level and a photon comes along with an energy equal to the difference
between the electron's energy and a lower energy. What will happen is that
the photon will stimulate the electron to fall into the lower energy state,
thereby emitting a photon. This is pictured below.
Figure 12.7:
Stimulated emission
 |
The emitted photon will have the same energy
as the original photon, and viewed as waves we will then have two
waves emerging from the atom in phase with the same frequency.
Such waves will constructively interfere, leading to a more intense
wave.
This is the principle behind the laser, which stands for
Light Amplification by Stimulated Emission of Radiation. In a laser
atoms are kept in an excited state by ``pumping'' the laser, and
some photons are inserted. This causes some atoms to undergo
stimulated emission, and the resulting photons cause other atoms to
undergo stimulated emission, leading to a chain reaction. The resultant
light is very intense and coherent (composed of one frequency), and
can be easily focused. Originally viewed as a curiosity, lasers
now are found in a wide variety of applications, including surgery,
precision cutting tools, CDROM readers, supermarket checkout stand
scanners, and holograms.





Next: Wave particle duality
Up: Bohr model
Previous: Absorption spectrum
modtech@theory.uwinnipeg.ca
1999-09-29
