




Next: Absorption spectrum
Up: Bohr model
Previous: Quantized orbits
Emission spectrum
Suppose an electron exists in a higher energy level (larger value of n).
Systems generally like to be in as low an energy state as possible,
and so the electron would fall into a lower energy state. However,
this process by itself would involve a loss of energy, and so energy
would not be conserved. To conserve energy, it was postulated that
a particle called a photon carry away the excess energy in this
transition, as pictured below.
Figure 12.4:
Photon emission
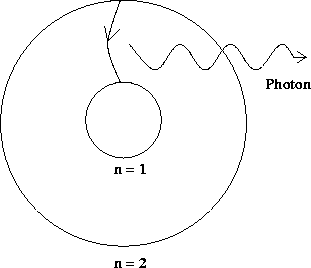 |
Note that the energy of this photon is fixed, as it must be equal to the
known difference in the energy levels of the electron transition.
It would be natural to identify this photon with the light found when atoms
emit light, and for this we need to know the frequency of the emitted
light. Another hypothesis was put forward relating the energy of the photon
to the frequency of the light wave:

where ``h'' is again Planck's constant.
Note that, for a given atom, there can be many types of transitions
from higher to lower energies, and thus many different energies of
emitted photons and, subsequently, many different frequencies of light waves.
Some of these are illustrated below.
Figure 12.5:
Possible transitions in the Bohr atom
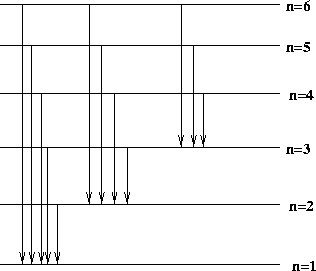 |
Transitions ending up at the n = 2 level give rise to photons in the
Balmer series, which is light in the visible part of the electromagnetic
spectrum. Transitions to the n = 1 level give rise to the Lyman series,
and to the n = 3 level give the Paschen series. Such series of
distinct spectral lines are observed experimentally when a gas such
as hydrogen is heated up - the added heat energy excites the electrons,
which subsequently fall into lower energy levels, emitting photons
of various energies and, thus, light of various frequencies. The observed
frequencies correspond very well to what is predicted in the Bohr
model.
This success of the Bohr model in explaining the emission spectra of
simple atoms gave birth to an intense, and still on-going, investigation
into what we now call quantum theory.





Next: Absorption spectrum
Up: Bohr model
Previous: Quantized orbits
modtech@theory.uwinnipeg.ca
1999-09-29

![]()