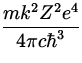Next: Modifications of the Bohr
Up: Atomic Physics
Previous: The Bohr Model
After its early success attempts were made to extend Bohr's model
to atoms other than hydrogen. The most successful models of this
type were those atoms with a single electron but with Z protons
in the nucleus; examples of these are singly ionized helium, doubly
ionized Lithium, and so on. In these atoms one simply replaces the
charge e of the proton in the hydrogen atom by the charge Ze of
the nucleus. Then, for example, the formula for the emission spectra
(28.14) gets modified to
which again agrees well with experiments for these atoms. However,
the success of this approach when applied to other atoms containing
two or more electrons is limited, mainly due to the complicated
nature of the multiple Coulomb interactions between electrons and protons,
and generally approximations or numerical techniques must be used.
www-admin@theory.uwinnipeg.ca
10/9/1997
![]()
![]()
![]()
![]()