![]()
Liquids on the other hand have no shape of their own. They take on the shape of whatever container they happen to be in. However, liquids, like solids, are difficult to compress. Finally, gases have neither a definite shape, nor density. They tend to fill whatever container they occupy, as long as the container is sealed. Otherwise the gases leak out and spread out as far as they can. All types of matter are made of molecules. The difference between solids, liquids and gases has to do with how tightly the molecules are held together by electromagnetic forces. In solids, the molecules are very tightly bound into rigid structures, whereas in liquids the binding forces are looser, allowing the molecules to slide over each other (flow). Gas molecules on the other hand have virtually no binding forces between them, so that they tend to get as far away from each other as their container allows.
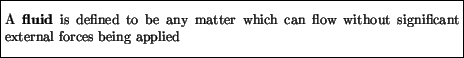
This definition can apply to either liquids or gases, but In this Chapter we will for simplicity assume that the fluids are essentially incompressible. Although this strictly speaking restricts us to liquids, most of our considerations will also apply, at least approximately, to gases under certain conditions. Thus, for example, we will be apply to apply our results to the air in our atmosphere.