 |
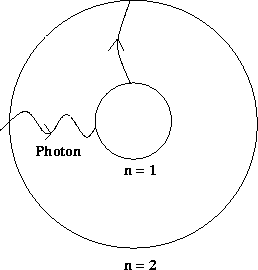
Such an effect can be seen by shining light of all different frequencies through a gas of a particular element. Some of the photons will be absorbed in the gas, and so will be missing in the light that emerges from the gas. What one will therefore see from the emerging light is an almost continuous band of frequencies, but with some frequencies missing corresponding to the absorbed photons.
As with the emission spectrum, each element has its own unique absorption spectrum, as the energy levels of different elements differ. Measuring the absorption spectrum can therefore be used to identify elements in an unknown substance by comparing it to the spectrum of known elements. Among other areas, this is a major technique used to identify elements in gaseous clouds in galaxies.